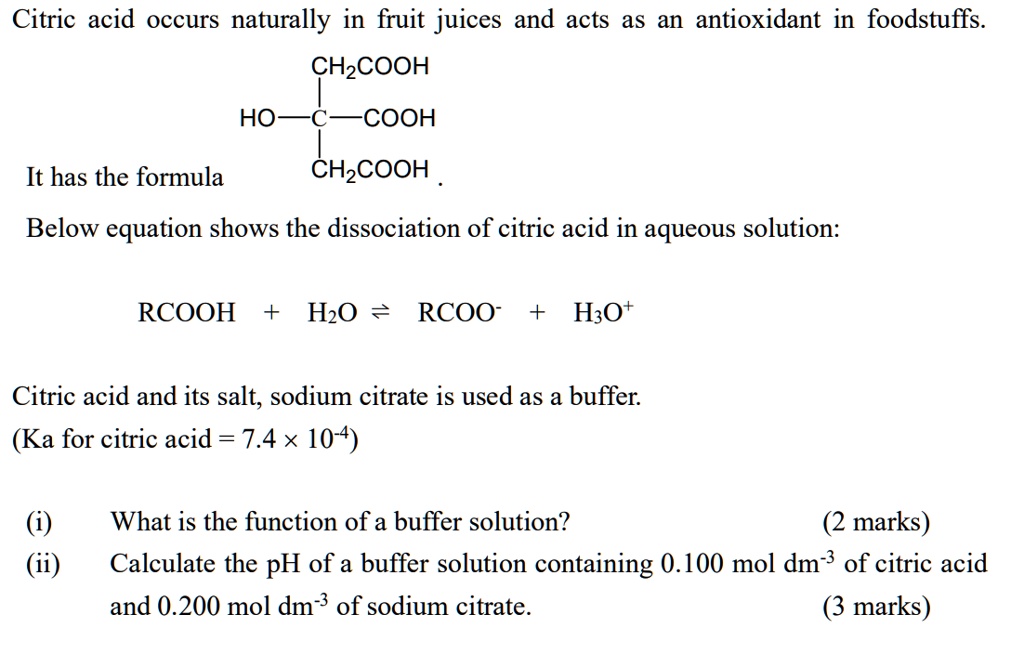
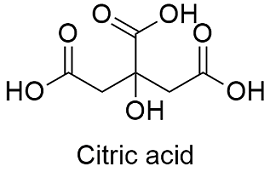
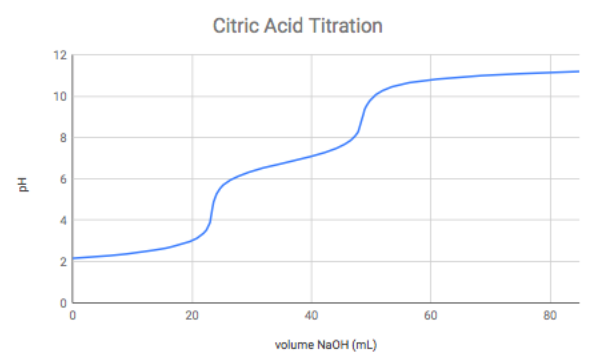
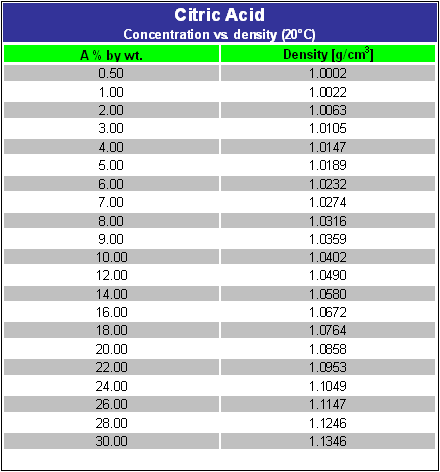
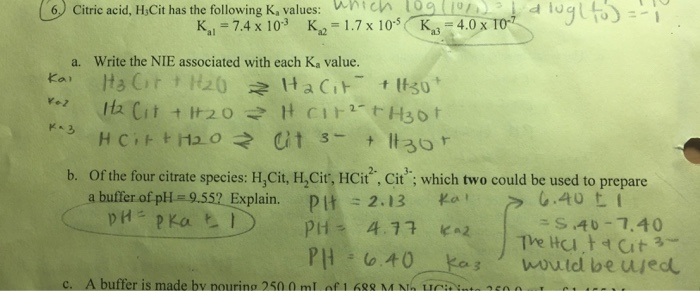SOLVED: Citric acid has 3 pKa values: 3.128, 4.761, 6.396. Calculate the Kf for the metal-EDTA complex: Number

Lemon juice normally has a `pH` of `2`. If all the acid the lemon juice is citric acid and there are - YouTube

The Effects of Combination of Citric Acid and Microbial Phytase on the Egg Quality Characteristics in Laying Hens

Amazon.com : Rani Citric Acid Powder, Food Grade (Limbu Ka Ful) 5oz (141g) PET Jar ~ Used for Cooking, Bath Bombs, Cleaning | Gluten Friendly | Indian Origin : Grocery & Gourmet Food

SOLVED:Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3) . (a) Calculate the pH of a 0.040M solution of citric acid. (b) Did you have to make